Ozone
What is ozone?
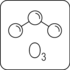
Ordinary oxygen is a gas molecule made of two oxygen atoms (O2). Ozone, on the other hand, is a gas molecule made of three oxygen atoms (O3).
How is ozone created?
The oxygen molecules (O2) are broken apart into two separate oxygen atoms. This happens when ultraviolet (UV) light hits the oxygen molecules or when electrical discharges (arcs, sparks) take place.
These two separated oxygen atoms are very reactive and tend to combine with other molecules in order to gain on stability. They achieve this by bonding with other oxygen molecules (O2) resulting in a molecule made of three oxygen atoms (O3), or what is called ozone.
Ozone remains unstable and naturally tends to decompose back into oxygen (O2) by reacting with virtually any substances: organic compounds, metals, plastics, odors, etc. This is what we call the oxidation process, or the chemical reaction between any elements and oxygen.
Ozone at the stratospheric level
Ozone in the stratosphere (20 to 50 km above the earth's surface) is naturally produced when UV-Light strikes oxygen molecules. It creates that way a layer which has the ability to absorb UV-light and protect us from its harmful radiations.
The "ozone hole" is when this ozone layer is becoming too thin to effectively block UV radiation. Several man-made substances destroy this layer, such as freon (used in air conditioners), fire extinguishers, insulating foams and solvents. These chemicals reach the upper layers of the atmosphere and are broken down by the sun's radiation, releasing chlorine and bromine atoms which take away one of the oxygen atoms of the ozone molecules to create other substances.
Ozone at the tropospheric level
Ozone is created at ground level when sun light and heat react with various pollutants from power and chemical plants, aerosol sprays (Chlorofluorocarbon or CFC's used as aerosol-spray propellants), air-conditioning units and fridges (freon gases), vehicle emissions, etc. Even some electric motors create ozone because of the arcs / sparks between the commutator and the brushes.
Ozone also has an odor
Ozone has a sharp, chlorine-like odor. It was named after the Greek verb "ozein" which means "to smell". It is noticeable after lightning, in confined areas in presence of strong electrical fields (i.e. high voltage transformers), but also near UV tubes and gas lighters as well as any device generating sparks.
Ozone effects and uses
On the positive side ozone attacks the cell walls of bacteria, decomposes mold, mildew, and odors, and is used as an air and water disinfectant. It also allows to extent the shelf life of food products. It is used in kitchens ventilation to remove odors and allows higher combustion rates in the industry.
Because of the high reactivity of ozone, only a few materials may be used like stainless steel (quality 316L), titanium, aluminum (as long as no moisture is present), glass, polytetrafluorethylene (PTFE), or polyvinylidene fluoride (Silicone). Viton (FKM) may be used with the restriction of constant mechanical forces and absence of humidity. Cracks, embrittlement and/or shrinkage are the usual consequences when elastomers are exposed to ozone.
Materials and their resistances to ozone
Aluminum : Fair
EPDM : Good to fair
Galv. Steel : Fair
Glass : Excellent
Natural rubber : Very Poor
Neoprene : Fair
Polyamide (PA) : Fair
Polyethylene : Good
PTFE : Excellent
Silicone : Excellent
STST 304/316 : Good
Steel (Mild) : Very Poor
Viton : Excellent (1)
Zinc : Poor
(1) Only when not subject to mechanical pressures.
Ozone and the METU Products
Ozone will attack the NBR gaskets and, in time, any galvanized and stainless-steel parts. One should take into consideration that other parameters might reduce the ozone resistant such as the presence of heat, pollutants, contaminants and the like.
Disclaimer
The above information serve as indications only and are provided without any guarantees of completeness or accuracy.